Quick Links: Principles | Specificity | Transport | Documentation | FAQs
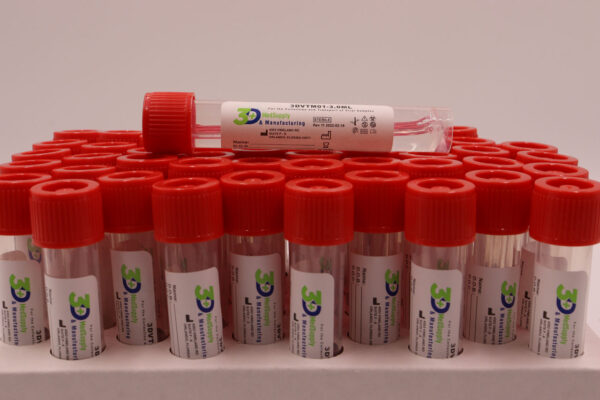
3DVTM01-3.0ML
The 3D MedSupply and Manufacturing VTM Tube is made in our ISO 5 clean rooms here in the USA with FDA Registration# 3020027409. We follow the strict CDC and FDA guidelines during the manufacturing process. The VTM is designed to facilitate the identification of all Viruses including, SARS-CoV-2, lnfiuenza A/B, RSV, as well as chlamydia and mycoplasma (bacteria) with any PCR technology by providing:
- Safe transport and preservation of the specimen
- Verified nucleic acid extraction and qRT-PCR, RT-PCR, qPCR, and RT-qPCR
Principles
The 3DVTM01-3.0ML Transit Tube contains a viral transport medium (VTM; Culture Media, Non-Propagating, Transport) intended to be inoculated with a Nasopharyngeal (NP), Oropharyngeal (OP), or Anterior Nares (AN) synthetic fiber swab specimens. It is then transported appropriately to the lab and analyzed with validated qRT-PCR, RT-PCR, qPCR and RT-qPCR assays for the detection of all Viruses, including severe acute respiratory syndrome (SARS-Cov-2), Influenza A/B, RSV, as well as chlyamydia and mycoplasma on humans.
Specificity
3DVTM01-3.0ML suppresses the growth of other bacteria and fungi that may be present in clinical samples from the human respiratory system.
Specimen
Nasopharyngeal, (NP), oropharyngeal (OP), or Anterior Nares (AN) swab.
Transport
Inoculated tubes should be transported within 72 hours after inoculation and maintained at 2-8°C.
Shelf Life
Can be stored at 2-8°C for up to 12 months and room temperature for 4 months without deterioration of performance, but not past the expiration date on the tube.
Availability
Contact us for availability.
U.S. Food & Drug – Administration

Establishment Name: 3D MedSupply & Manufacturing,LLC | FL/USA
Registration Number: 3020027409?
Current Registration Yr: 2022
Culture Media, Non-Selective And Non-Differential – 3DSWB01 | Manufacturer
Transport Medium, Notified Per The Vtm Guidance – 3DVTM01-1.5ML, 3DVTM01-3.0ML | Manufacturer
Made in the USA
Manufactured daily

Ready to Ship
In stock and ready to order

Viral Transport Medium
3DVTM01 Transport Medium Tube is for on-site collection and transport to the testing laboratory of human clinical specimens.
FAQs
What exactly is transported in the VTM?
The 3DVTM01 Transit Tube contains a viral transport medium (VTM) intended to be inoculated with nasopharyngeal (NP) or oropharyngeal (OP) synthetic fiber swab specimens for transport to the lab.
The 3DVTM01 Transport Medium is designed for specimen preservation in preparation for analysis with validated qRT-PCR assays to detect severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that causes COVID-19 disease in humans.
This is CDC-approved media for COVID-19 sample collection and transport. 3DVTM01 has been validated for stable transport of SARS-CoV-2 only.
How was the 3DVTM01 validated?
Validated by our internal R&D team using qRT-PCR. Validation results confirmed that 3DVTM01 maintained the viability of the RNA for 72 hours post-sample collection (when stored at 2-8°C). Cell lysate of SARS-Cov-2 infected cells were used for validation (not live virus).
Is shipping ambient temperature or cold?
3DVTM01 is shipped ambient temperature, storage conditions should be followed on arrival.
Can you provide a full validation report?
3D MedSupply & Manufacturing can provide the validation report per request only.
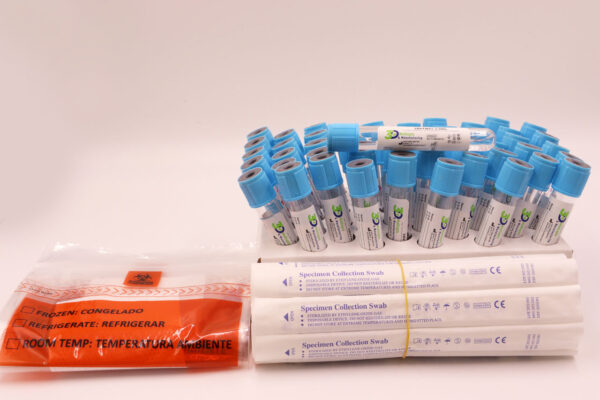
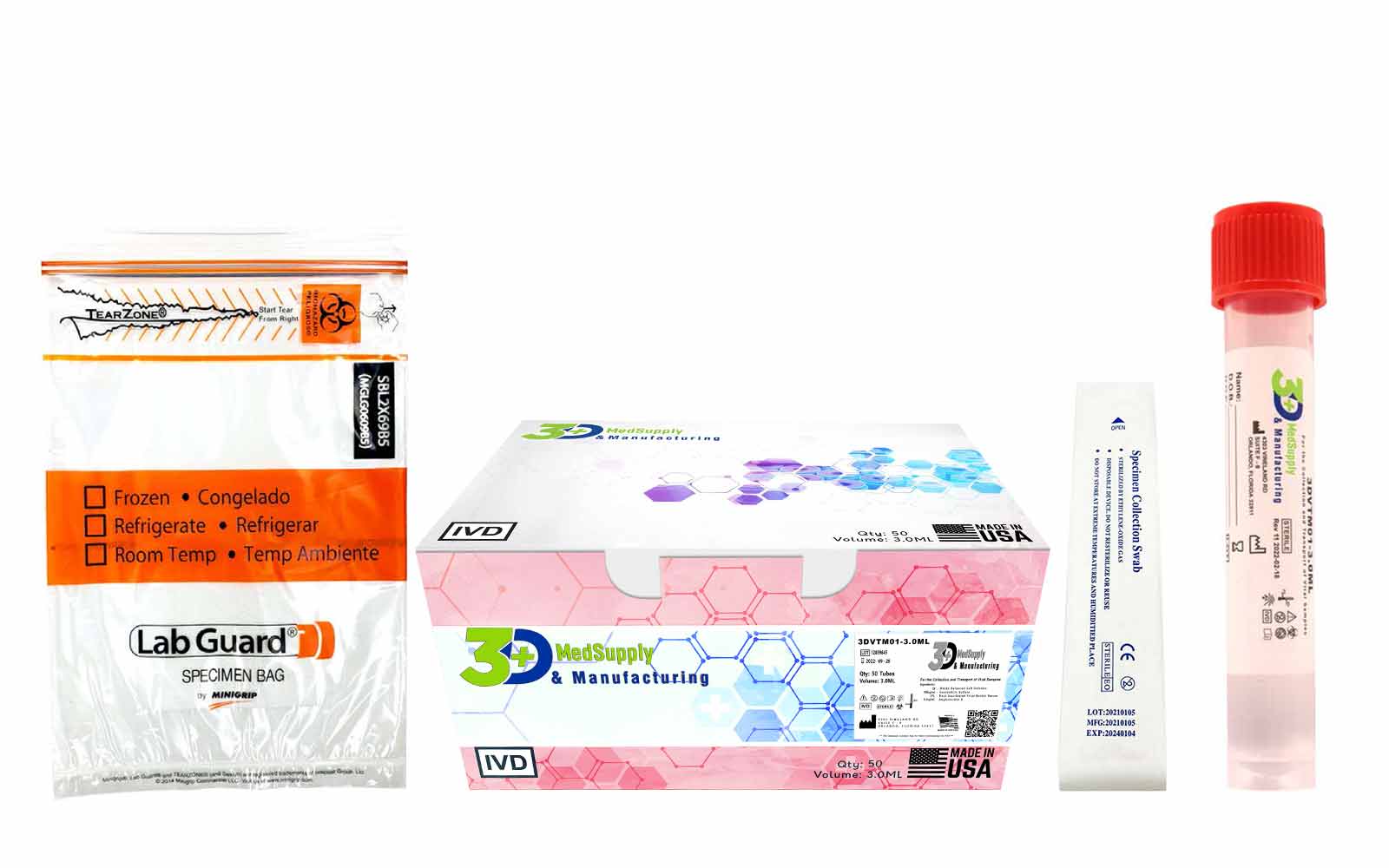
What does each box contain?
- (50) Sterile 3ml VTM tubes
- (50) Sterile Swabs
- (50) Biohazard Bags
Contact us for more information
Request Free Sample Kits
Fill out the form to get in touch with our team to order sample kits.